Home » Products » Pharmaceutical Raw Materials » Medicine Raw Materials » Vardenafil Hydrochloride CAS 224785-91-5 Whatsapp/signal/wechat: +86 15972166960 Wickr Me: Bellachen
Vardenafil Hydrochloride CAS 224785-91-5 Whatsapp/signal/wechat: +86 15972166960 Wickr Me: Bellachen
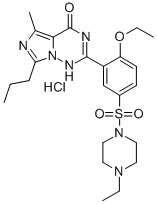
Product Name: Vardenafil hydrochloride
CAS: 224785-91-5
MF: C23H33ClN6O4S
MW: 525.06
EINECS: 606-590-1
Product Categories: Intermediates & Fine Chemicals;Pharmaceuticals;Vardenafil
Mol File: 224785-91-5.mol
CAS: 224785-91-5
MF: C23H33ClN6O4S
MW: 525.06
EINECS: 606-590-1
Product Categories: Intermediates & Fine Chemicals;Pharmaceuticals;Vardenafil
Mol File: 224785-91-5.mol
| Availability: | |
|---|---|
| Quantity: | |
- 224785-91-5
- Hanhong
- Whatsapp/signal/wechat: +86 15972166960 wickr me: bellachen
 |
| Vardenafil hydrochloride Chemical Properties |
| Melting point | 214-216°C |
| Vardenafil hydrochloride Usage And Synthesis |
| Description | Vardenafil was the second agent to be marketed and had the advantage that its onset time was not reduced by taking the medication on a full stomach . It is 30 times more potent as an inhibitor of PDE5 (mean IC50, 3.9 nM) than sildenafil and 10 times more potent than tadalafil, with a greater selectivity (>1,000 times) for human PDE5 than for human PDE2, PDE3, and PDE4 and moderate selectivity (>80 times) for PDE1. The PDE inhibitory selectivity and both the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Vardenafil specifically inhibited the hydrolysis of cGMP by PDE5, with an IC50 of 0.7 nM (sildenafil 6.6 nM). The IC50 of vardenafil for PDE1 was 180 nM, for PDE6 11 nM, and for PDE2, PDE3 and PDE4 more than 1,000 nM. |
| Chemical Properties | White to Off-White Cyrstalline Solid |
| Uses | A selective phsphodiesterase type 5 (PDE5) inhibitor |
| Uses | A phsphodiesterase 5 inhibitor. |
| General Description | Vardenafil, 4-[2-ethoxy-5-(4-ethylpiperazin-1-yl)sulfonyl-phenyl]-9-methyl-7-propyl-3,5,6,8-tetrazabicyclo[4.3.0]nona-3,7,9-trien-2-one(Levitra), was the second PDE5 introduced in the U.S. market.The metabolism of vardenafil is primarily by CYP3A4.As such, concomitant use of CYP3A4 inhibitors such as ritonavir,indinavir, ketoconazole, as well as moderate CYP3Ainhibitors such as erythromycin typically results in significantincreases of plasma levels of vardenafil. |
 |
| Vardenafil hydrochloride Chemical Properties |
| Melting point | 214-216°C |
| Vardenafil hydrochloride Usage And Synthesis |
| Description | Vardenafil was the second agent to be marketed and had the advantage that its onset time was not reduced by taking the medication on a full stomach . It is 30 times more potent as an inhibitor of PDE5 (mean IC50, 3.9 nM) than sildenafil and 10 times more potent than tadalafil, with a greater selectivity (>1,000 times) for human PDE5 than for human PDE2, PDE3, and PDE4 and moderate selectivity (>80 times) for PDE1. The PDE inhibitory selectivity and both the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Vardenafil specifically inhibited the hydrolysis of cGMP by PDE5, with an IC50 of 0.7 nM (sildenafil 6.6 nM). The IC50 of vardenafil for PDE1 was 180 nM, for PDE6 11 nM, and for PDE2, PDE3 and PDE4 more than 1,000 nM. |
| Chemical Properties | White to Off-White Cyrstalline Solid |
| Uses | A selective phsphodiesterase type 5 (PDE5) inhibitor |
| Uses | A phsphodiesterase 5 inhibitor. |
| General Description | Vardenafil, 4-[2-ethoxy-5-(4-ethylpiperazin-1-yl)sulfonyl-phenyl]-9-methyl-7-propyl-3,5,6,8-tetrazabicyclo[4.3.0]nona-3,7,9-trien-2-one(Levitra), was the second PDE5 introduced in the U.S. market.The metabolism of vardenafil is primarily by CYP3A4.As such, concomitant use of CYP3A4 inhibitors such as ritonavir,indinavir, ketoconazole, as well as moderate CYP3Ainhibitors such as erythromycin typically results in significantincreases of plasma levels of vardenafil. |