Home » Products » Pharmaceutical Raw Materials » Medicine Raw Materials » Dapoxetine Hydrochloride CAS 129938-20-1 Whatsapp/signal/wechat: +86 15972166960 Wickr Me: Bellachen
Dapoxetine Hydrochloride CAS 129938-20-1 Whatsapp/signal/wechat: +86 15972166960 Wickr Me: Bellachen
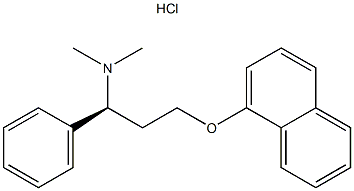
Product Name: Dapoxetine hydrochloride
Synonyms: DL-Dapoxteine HCL;N,N-DiMethyl-1-phenyl-3-(1-naphthalenyloxy)propanaMinehydrochloride;(S)-N,N-Dimethyl-3-(naphthalen-1-yloxy)-1-phenylpropan-1-amine hydrochloride;Dapoxetine/Dapoxetine HCl/Dapoxetine hydrochloride;LY-210448 hydrochloride;Priligy;PRILIGY;LY-210448 HYDROCHLORIDE;(S)-N,N-dimethyl-3-
CAS: 129938-20-1
MF: C21H23NO·HCl
MW: 341.88
EINECS: 640-411-8
Product Categories: Amines;Aromatics;Inhibitors;Dapoxetine Hydrochloride;Intermediates & Fine Chemicals;Pharmaceuticals
Mol File: 129938-20-1.mol
Synonyms: DL-Dapoxteine HCL;N,N-DiMethyl-1-phenyl-3-(1-naphthalenyloxy)propanaMinehydrochloride;(S)-N,N-Dimethyl-3-(naphthalen-1-yloxy)-1-phenylpropan-1-amine hydrochloride;Dapoxetine/Dapoxetine HCl/Dapoxetine hydrochloride;LY-210448 hydrochloride;Priligy;PRILIGY;LY-210448 HYDROCHLORIDE;(S)-N,N-dimethyl-3-
CAS: 129938-20-1
MF: C21H23NO·HCl
MW: 341.88
EINECS: 640-411-8
Product Categories: Amines;Aromatics;Inhibitors;Dapoxetine Hydrochloride;Intermediates & Fine Chemicals;Pharmaceuticals
Mol File: 129938-20-1.mol
| Availability: | |
|---|---|
| Quantity: | |
- 129938-20-1
- Hanhong
- Whatsapp/signal/wechat: +86 15972166960 wickr me: bellachen
| Dapoxetine hydrochloride Chemical Properties |
| Melting point | 175-1790C |
| alpha | D +135.78° (c = 2.18 in methanol) |
| storage temp. | -20°C Freezer |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white |
| optical activity | [α]/D +125 to +135°, c = 1 in methanol |
| Merck | 14,2821 |
| CAS DataBase Reference | 129938-20-1(CAS DataBase Reference) |
| Dapoxetine hydrochloride Usage And Synthesis |
| Description | PE is the most common form of male sexual dysfunction affecting 20-40% of men globally at some point in their lives. Ejaculation is a reflex comprising different sensory pathways, motor centers, and nerve pathways. This ejaculatory reflex has been shown to be controlled primarily by serotonin and dopamine. Dapoxetine is a short-acting SSRI. It is differentiated from the existing SSRI treatments for PE by the fact that it can be administered on an as-needed basis. In healthy subjects, dapoxetine is rapidly absorbed after oral administration with a peak plasma concentration (Tmax) occurring between 1.4 and 2 h. Dapoxetine also showed statistically significant improvements in perceived control over ejaculation, PE-related personal distress, and other patient-reported outcomes in all five trials. Dapoxetine treatment was generally well tolerated, with low incidences of discontinuation syndrome, sexual dysfunction, and treatment-emergent mood symptoms. The most common adverse events with dapoxetine included nausea, diarrhea, headache, dizziness, and somnolence. |
| Chemical Properties | Off-white Cryst |
| Originator | Lilly (US) |
| Uses | Selective serotonin reuptake inhibitor (SSRI) |
| Uses | Dapoxetine hydrochloride is a short-acting novel selective serotonin reuptake inhibitor marketed for the treatment of premature ejaculation in men. Premature ejaculation (PE) is the most common male sexual disorder, estimated to affect up to 30% of men. D |
| Uses | A selective serotonin reuptake inhibitor |
| Brand name | Priligy |
| Chemical Synthesis | Dapoxetine can be synthesized in four chemical steps starting from R-1phenyl- 1,3-propanediol via selective tosylation of the primary hydroxy group with p-toluenesulfonyl chloride, triethylamine and 4-(dimethylamino) pyridine (DMAP), and subsequent condensation with 1naphthol by means of sodium- or lithium hydroxide to yield R-3-(1naphthyloxy)- 1-phenylpropanol as the key intermediate. Conversion of the alcohol group to the corresponding mesylate with methanesulfonyl chloride, triethylamine, and DMAP, followed by treatment with dimethylamine affords dapoxetine, which is then acidified to its hydrochloride salt. |
| Dapoxetine hydrochloride Chemical Properties |
| Melting point | 175-1790C |
| alpha | D +135.78° (c = 2.18 in methanol) |
| storage temp. | -20°C Freezer |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white |
| optical activity | [α]/D +125 to +135°, c = 1 in methanol |
| Merck | 14,2821 |
| CAS DataBase Reference | 129938-20-1(CAS DataBase Reference) |
| Dapoxetine hydrochloride Usage And Synthesis |
| Description | PE is the most common form of male sexual dysfunction affecting 20-40% of men globally at some point in their lives. Ejaculation is a reflex comprising different sensory pathways, motor centers, and nerve pathways. This ejaculatory reflex has been shown to be controlled primarily by serotonin and dopamine. Dapoxetine is a short-acting SSRI. It is differentiated from the existing SSRI treatments for PE by the fact that it can be administered on an as-needed basis. In healthy subjects, dapoxetine is rapidly absorbed after oral administration with a peak plasma concentration (Tmax) occurring between 1.4 and 2 h. Dapoxetine also showed statistically significant improvements in perceived control over ejaculation, PE-related personal distress, and other patient-reported outcomes in all five trials. Dapoxetine treatment was generally well tolerated, with low incidences of discontinuation syndrome, sexual dysfunction, and treatment-emergent mood symptoms. The most common adverse events with dapoxetine included nausea, diarrhea, headache, dizziness, and somnolence. |
| Chemical Properties | Off-white Cryst |
| Originator | Lilly (US) |
| Uses | Selective serotonin reuptake inhibitor (SSRI) |
| Uses | Dapoxetine hydrochloride is a short-acting novel selective serotonin reuptake inhibitor marketed for the treatment of premature ejaculation in men. Premature ejaculation (PE) is the most common male sexual disorder, estimated to affect up to 30% of men. D |
| Uses | A selective serotonin reuptake inhibitor |
| Brand name | Priligy |
| Chemical Synthesis | Dapoxetine can be synthesized in four chemical steps starting from R-1phenyl- 1,3-propanediol via selective tosylation of the primary hydroxy group with p-toluenesulfonyl chloride, triethylamine and 4-(dimethylamino) pyridine (DMAP), and subsequent condensation with 1naphthol by means of sodium- or lithium hydroxide to yield R-3-(1naphthyloxy)- 1-phenylpropanol as the key intermediate. Conversion of the alcohol group to the corresponding mesylate with methanesulfonyl chloride, triethylamine, and DMAP, followed by treatment with dimethylamine affords dapoxetine, which is then acidified to its hydrochloride salt. |